Bromine Atomic Mass
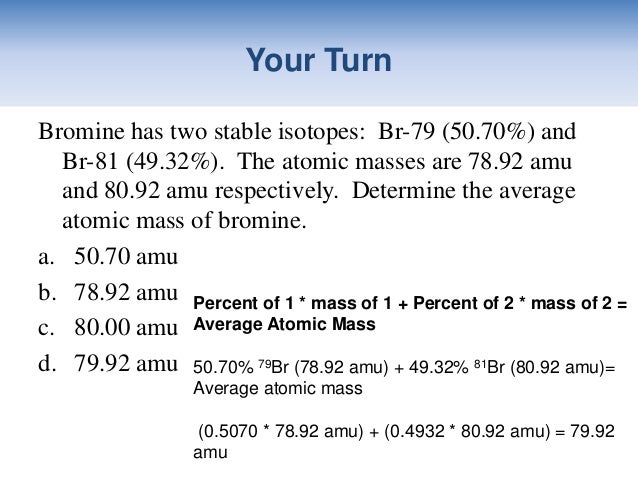
What is the average atomic mass of bromine? One isotope of bromine has an atomic mass of 78.92amu and a relative abundance of 50.69%. The other major isotope of bromine has an atomic mass of 80.92amu and a relative abundance of 49.31%.

1 Answer
Bromine is the only nonmetallic liquid element. It is a heavy, mobile, reddish-brown liquid, volatilizing readily at room temperature to a red vapor with a strong disagreeable odor, resembling chlorine, and having a very irritating effect on the eyes and throat; it is readily soluble in water or carbon disulfide, forming a red solution, is less active than chlorine but more so than iodine; it. Atomic Data for Bromine (Br) Atomic Number = 35 Atomic Weight = 79.904 Reference E95: Isotope: Mass: Abundance. 49.31%: 3/2 +2.2706: Br I Ground State 1. Element Bromine (Br), Group 17, Atomic Number 35, p-block, Mass 79.904. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. It provides atomic mass, mass excess, nuclear binding energy, nucleon separation energies, Q-values, and nucleon residual interaction parameters for atomic nuclei of the isotope Br-81 (Bromine.

Bromine Atomic Mass Average
The average atomic mass of Br is 79.91 u. Zee smile live.
There are two ways to solve this problem. The indecision band.
Bromine Atomic Mass Of Isotopes
Method 1
Let's assume we have 10 000 atoms of Br. Then 5069 atoms will have mass 78.92 u, and 4931 atoms will have mass 80.92 u.
Mass of the 5069 atoms = 5069 × 78.92 u = 400 000 u
Mass of the 4931 atoms = 4931 × 80.92 u = 399 000 u
Mass of 10 000 atoms = 799 100 u
Average mass of an atom =
Bromine Atomic Mass Rounded
Method 2

The average mass is the mass of each isotope multiplied by its percentage. Blues brothers torrent. Thus,
Average mass = 50.69 % × 78.92 u + 49.31 % × 80.92 u = 40.00 u + 39.90 u = 79.91 u

The second method is easier, but it doesn't explain intuitively why the answer is the average atomic mass.
Here is a video which summarizes the steps needed to calculate average atomic mass.
Related questions
